This continues a discussion I began here. I think the point is established that if you want to see fine detail, the solution is not a smaller "eye" but a larger one. But I have an error to correct.
In the prior post, I stated the Rayleigh criterion for the resolution of a diffraction-limited optical system, which is based on the Airy formula
x = 1.22 λl/d
where l/d is the focal ratio, such as 4:1 for an f/4 system.
I neglected to mention that this formula is only valid if the light path between the object and the viewing lens is vacuum (or air, to a close approximation). In the case of high-powered optical microscopes, the space between the object and the objective lens is filled with an oil that has the same index of refraction as the glass of the slide and of the lens, typically about 1.5. The symbol for refractive index is n, so in an optical microscope with an oil-immersion objective, the formula is
x = 1.22 λl/nd
This replacement of λ with λ/n resolves the difference I'd stated between the Rayleigh criterion and the Abbe limit: 0.866/1.5 = 0.58, which means that an oil-immersed lens with N.A. = 1.4 (f/0.71) will resolve details as small as 0.58λ, which is 0.35µ for incandescent light (effective λ = 0.6µ) and 0.28µ for daylight (effective λ = 0.48µ).
My calculations for ultraviolet viewing remain unchanged because immersion oils absorb UV light.
Now, before we consider even smaller wavelengths, there is a technology that can resolve very small details for certain objects, using visible light. That is Near-field Scanning Optical Microscopy, or NSOM.
A very (very!) smooth sample can be scanned with a narrow glass fiber tip, just a few nanometers across. As long as the fiber tip is within a few nm of the surface being scanned, the spot of light will effectively be very much smaller than a wavelength. In practice, resolutions of around 10nm have been achieved. This is fifty times as good as imaging with lens optics. This technology is not for the faint of heart or poverty of pocketbook. Just drawing a glass fiber so it necks down to 3-5 nm diameter is a challenging task. Handling it to mount it and use it without breaking it…tricky. If you don't need the optical response, electron microscopes are cheaper, both SEM and TEM.
The concept to grasp for what follows is that of disturbance. We don't think of it this way, but even using visible light to view an object disturbs the object. The way light is reflected requires disturbing the outer electrons of a substance, which either absorb or re-emit the photons that bang into them. Visible photon energies are low, however, and don't typically cause atoms to be shifted out of position. Even x-rays and electrons a thousand times as energetic as visible photons seldom force atoms to jump aside.
But the electron bombardment, in particular, cause a disturbance in electron orbits. When a high-powered electron microscope is used to image atoms, we are seeing them slightly distorted, because what we are seeing is an image of the threshold position of the electron cloud around each atom's nucleus, at some equilibrium position as blown by the "wind" of electrons we are viewing with.
Is viewing the atoms, or their outer electron clouds, the ultimate achievable? Not at all. Hitting a small enough sample with a sufficiently dense electron beam, we can strip off the outer electrons, ionizing the atoms in the sample, but the ions will quickly neutralize by grabbing electrons from a cloud that builds up around the sample. Ion imaging may have its place, but I haven't heard of it being used. Nonetheless, there may be a way to use such a technique to probe various electron orbitals. X-rays are more typically used to do that, however, and the technology is well understood. We won't go further with that here.
Another reason for disturbing atoms enough to ionize them is related to probing the mechanics of the structure of a single atom's electron cloud. In this small realm, the order and spacing of each electron's orbital cloud are governed by quantum mechanics. For such studies to be meaningful, the atoms need to be isolated, so charged gases and plasmas are used. Atoms so disturbed emit and absorb electromagnetic radiation of all kinds, so this is the realm of spectroscopy, which covers a vast range of energies, from quite low (medium infrared starting at about 3µ) through visible and ultraviolet to x-rays with wavelengths of less than 1nm and energies of 100 KeV or more.
The next barrier is the atomic nucleus. What does it take to disturb it enough to learn about its innards? Ernest Rutherford began to show the way by bombarding gold foil with alpha particles, which are totally ionized helium nuclei. He was throwing one kind of nucleus at another, though he didn't know it. The α particles had an energy near 4 MeV, and when one came close to a gold nucleus, it bounced right back, or to the side. So four million volts isn't enough to do more than get a rough fix on the location of a nucleus.
As it turns out, once the proton was discovered (it is a bare hydrogen nucleus), it was found to weigh about 1,800 times as much as an electron. It takes many MeV to do more than make a proton bounce around like a smacked ping pong ball. Today's particle physics, developed since the 1950s, is in the business of finding out how the components of protons work together. It takes millions of MeV to have much chance of cracking open a proton (AKA p+), and its energetic components are very shy, splitting into showers of other particles within 10-24 second.
The diameter of a nucleus is so small that, it has been often written, its size compared to the electron cloud is similar to a grain of sand hovering at the center of a cathedral or airplane hanger. An iron atom is about 10-10m in diameter, and its nucleus is a bit smaller than 10-13m, a thousand times smaller.
How big a machine is required to "see" details this small, and smaller? As it turns out, the finer the details you want to see, the bigger the machine it takes. Particle Accelerators are the tool we need to produce a probe that can see inside a proton. Accelerators are of two types: linear and circular. An ordinary TV set, and a small electron microscope, are both accelerators of the linear type, that accelerate electrons to energies of 30-50 KeV. Early accelerators that fit in a lab with a high ceiling, known as Cockcroft-Walton accelerators, could produce electrons with energies of a few MeV, and they are 4-5m tall. Both these kinds of accelerator are one-push types: make lots of volts and let electrons "fall" from one end to the other.
Higher energies are produced by letting the accelerated electrons fly through a hole and giving them another push with radio waves. The biggest of these, the Stanford Linear Accelerator (SLAC) imparts energies up to 50 GeV to electrons. But linear is a one-shot kind of technology. Once the electrons fly out the end, you're done with them. Much higher energies are had by running particles in circles, so you can push them over and over again, then divert them to hit your target.
The modern tool for this is the synchrotron. The old Cosmotron was 23m in diameter and imparted a bit more than 3 GeV to protons. The main synchrotron that accelerates electrons, the LEP, gives them the same energy as SLAC, but it can run beams in opposite directions so as to crash them head-on, for more energetic collisions. It can do the same with positrons, or with positrons one way and electrons the other. Lotsa possibilities! But protons, being 1,800 times as heavy, achieve much higher energies. The largest proton accelerator, the LHC (which operated briefly late last year, and is now being repaired!), should achieve 7 TeV (million MeV) per proton. But the LHC is designed to accelerate heavy nuclei containing many protons and neutrons, for purposes of learning more about how the quarks and gluons that make up a whole nucleus interact. The LHC is 8,500m in diameter, or 8km.
Putting the few figures I've given above with others, I produced this chart:
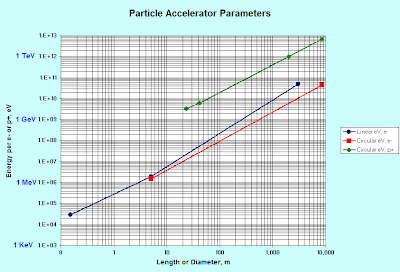 In the legend, p+ is for proton and e- is for electron. For electrons, getting to a certain energy takes about the same size machine, whether linear or circular. Circular machines have losses, however, because turning the electrons in a circle causes bursts of electromagnetic energy to leak away. This accounts for the somewhat different scale between the red and blue lines.
In the legend, p+ is for proton and e- is for electron. For electrons, getting to a certain energy takes about the same size machine, whether linear or circular. Circular machines have losses, however, because turning the electrons in a circle causes bursts of electromagnetic energy to leak away. This accounts for the somewhat different scale between the red and blue lines.The green line shows proton energies in a few synchrotrons. The straightness of the line allows us to determine a formula, for gaining more energy if our investigations in the future require it:
Emax = 53.2 MeV x Diameter1.3, for Diameter in meters.
Though we are producing particles with TeV scale energies, more is always desired. The 10-24s time scale of quark-gluon interactions requires a wavelength of 3x10-16m, or a particle energy of 41 GeV. In practice, it takes many times this energy, most of which is used to create "resonance" particles that spall away, so some is left over to push a couple of quarks a few trillionths of a meter apart so you can get a reaction you can measure.
How much energy is "enough"? Some Cosmic rays are more energetic than the output of the biggest accelerator we could build on earth. Let us use the formula above to calculate the energy from an accelerator 14,700km in diameter: we get 1.1x1017 eV, or 110,000 TeV. The most powerful cosmic ray so far detected had an energy of 3x1018 eV, and an accelerator to produce such particles would need to be 4 million km in diameter.
Until new technologies allow more efficient circular accelerators, then, we are limited to some thousands of TeV. The wavelength of ~100,000 TeV particles is as short as 10-22m, and they can interact on time scales as brief as 10-30 second. And that's the finest resolution achievable from machines that fit onto the surface of the earth.



No comments:
Post a Comment